Understanding Electrolytic Cells: Types, Components, and Applications
An electrolytic cell is an essential electrochemical device that drives a non-spontaneous redox reaction using electrical energy. Unlike a galvanic (or voltaic) cell, which generates electricity from spontaneous chemical reactions, an electrolytic cell consumes electricity to initiate a chemical transformation—often used in processes like electroplating, water electrolysis, and CO₂ reduction.
Key Components of an Electrolytic Cell
Every electrolytic cell consists of a few fundamental parts:
- Anode (+): The site of oxidation.
- Cathode (−): The site of reduction.
- Electrolyte: A molten or aqueous ionic solution that facilitates ion transport.
- DC Power Supply: Provides the energy required to drive the reaction.
- External Circuit: Connects the electrodes to the power source and enables electron flow.
These components work together to enable precise control over electrochemical reactions, allowing researchers to tune the experimental conditions for specific applications.
Types of Electrolytic Cells
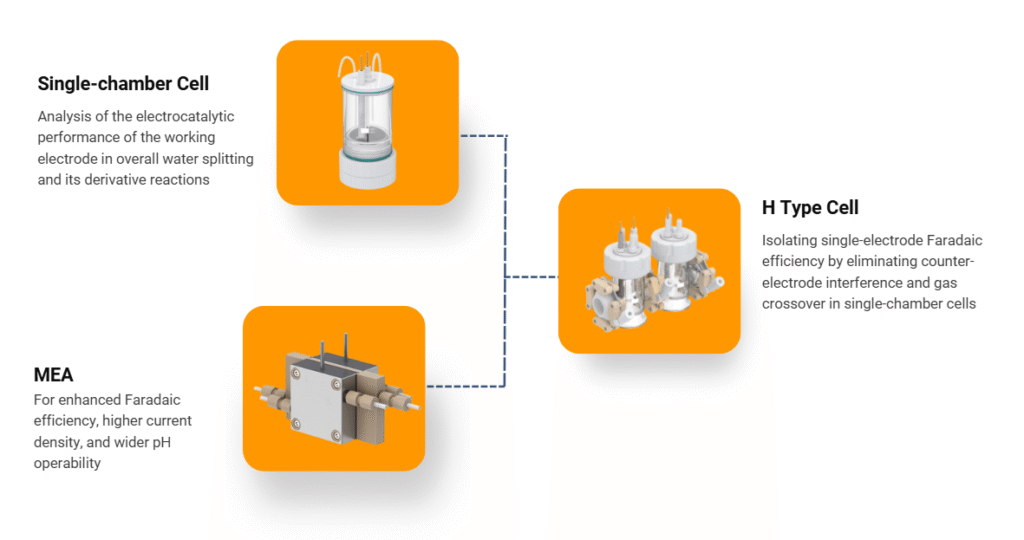
Depending on the application and experimental design, electrolytic cells come in several configurations:
- Single-Chamber Cells
- Two-Electrode System: Often used for general electrolysis experiments where gas separation is not critical.
- Three-Electrode System: Includes a reference electrode for accurate potential control—commonly used in electrochemical research.
- H-Type Cells
- Designed with two separate chambers connected by a salt bridge or proton exchange membrane.
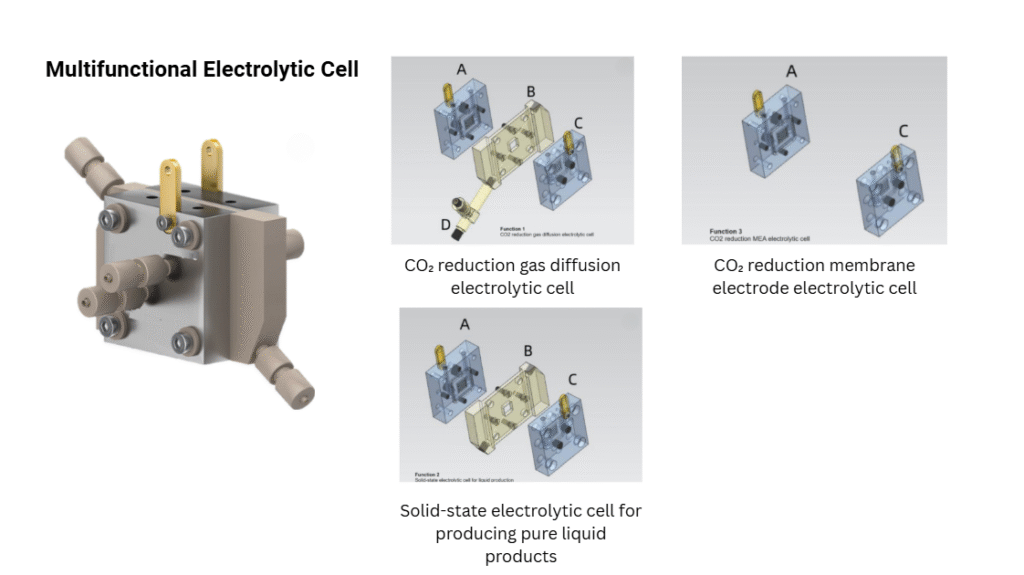
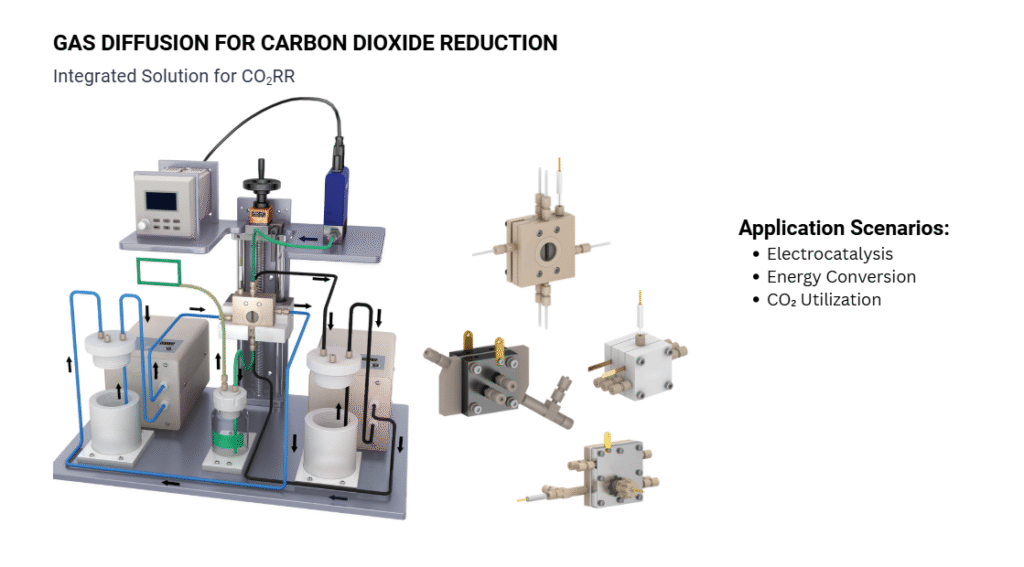
- Ideal for experiments requiring product separation, such as CO₂ reduction and oxygen/hydrogen evolution reactions.
- Available in two-electrode and three-electrode configurations.
- Membrane Electrode Assembly (MEA)
- Integrates electrode and membrane layers into a compact stack.
- Widely used in fuel cell research and water electrolysis applications for high-efficiency performance.
Common Applications
- Water Electrolysis: Splitting water into hydrogen and oxygen using an electric current.
- CO₂ Reduction: Converting carbon dioxide into value-added chemicals or fuels.
- Fuel Cells: Operating in reverse to produce electricity from hydrogen and oxygen.
Each setup offers unique advantages depending on the research goals—whether it’s gas collection, product analysis, or improving reaction kinetics.
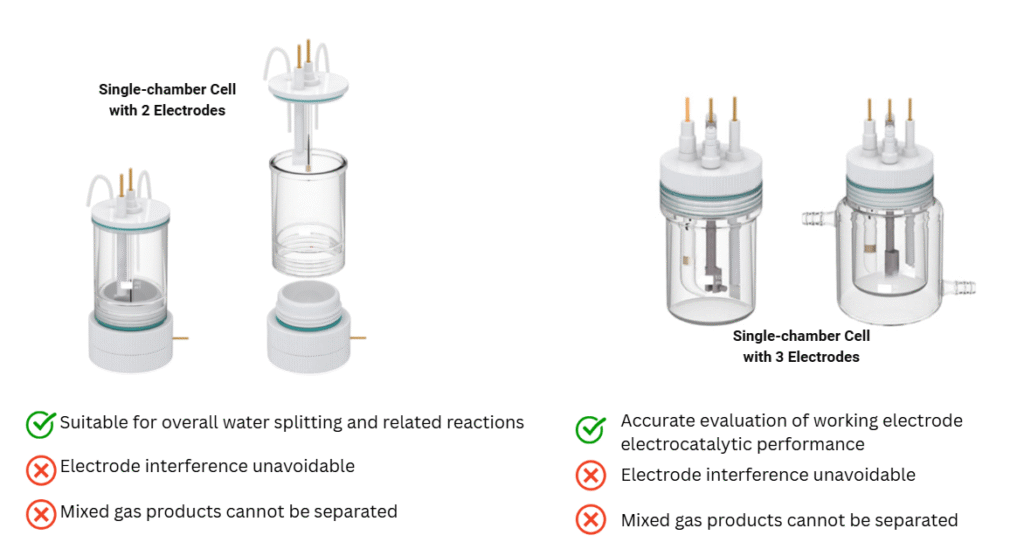
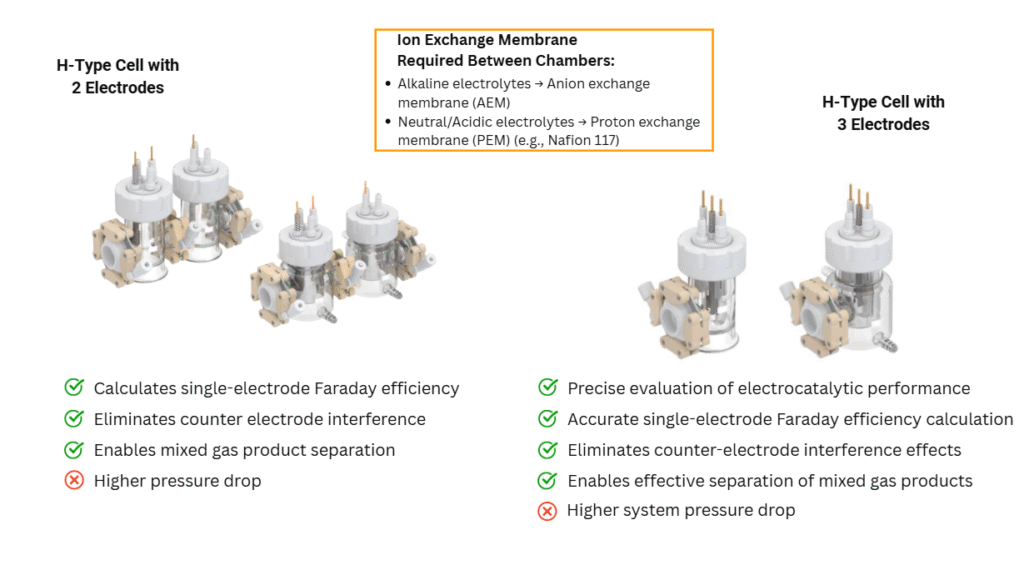
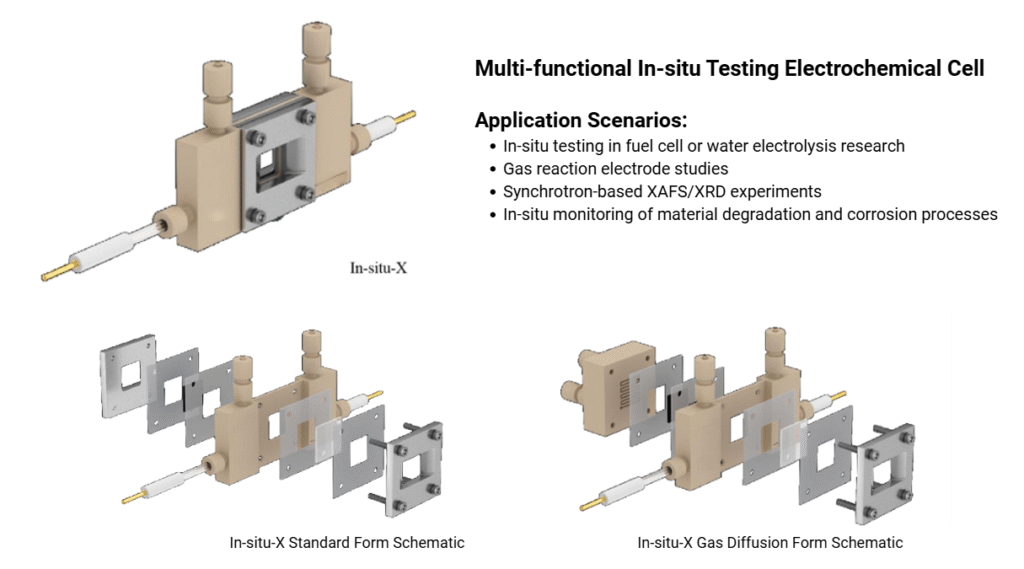
Choosing the right electrolytic cell setup is crucial for accurate results and reproducible data. Whether you’re working on sustainable energy, electrocatalysis, or materials research, understanding the structure and function of electrolytic cells will enhance your experimental outcomes.
Other Blog Posts You Might Like
Build, Print, Cure: Materials That Make 3D Printing Work
As 3D bioprinting continues to advance rapidly, the performance and functionality of materials have become…
Read moreANR Operations Team: The Unseen yet Essential Support Behind Your Laboratory Needs
At ANR, we believe that providing products isn’t enough—it’s the complete, reliable support we offer…
Read moreThe Core Logic Behind Electrolyte Formulation Development
An Engineering Perspective on Performance Trade-offs Electrolyte formulation is often described as a process of…
Read more


