Description
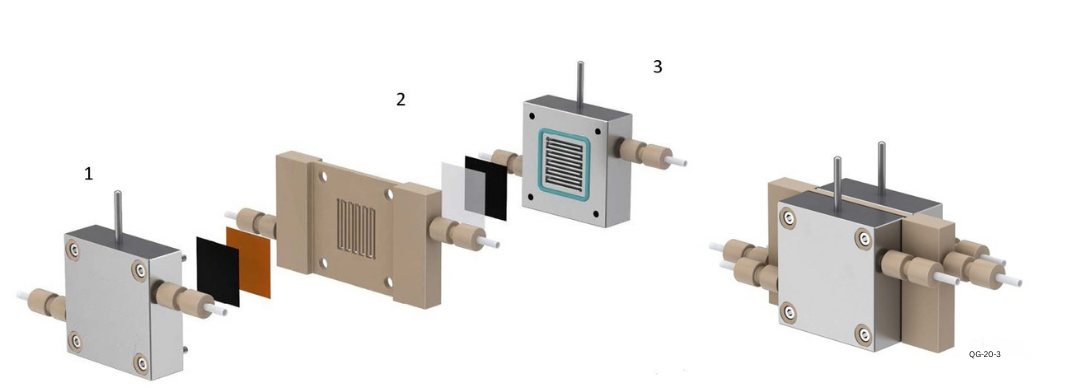
The QG-20-3 represents an advancement over the QG-20-1, specifically enhancing the design of the second electrode plate 2 by incorporating a fluid-dynamic friendly serpentine channel. The overall architecture of the solid-state electrolyte cell embraces a sandwich structure, establishing a robust gas-solid interface using solid electrolyte in lieu of liquid electrolyte. This innovative approach prevents the introduction of unwanted anions/cations, ensuring the direct production of high-purity liquid product solutions.
Features:
• Electrode bodies 1 and 3 serve as bipolar plates, featuring inner serpentine gas flow channels. This design effectively elevates the concentration of gaseous reactants on the catalyst surfaces, achieving high current density.
• Between electrode bodies 1 and 2, a double-layer structure comprising an anion membrane (dioxide material) and a gas diffusion layer (loaded with anode catalyst) is utilized to establish the gas-solid interface and facilitate anion transport (Note: hot pressing of catalyst material is required for sufficient contact). Similarly, between electrode bodies 2 and 3, a double-layer structure with a cation membrane (Nafion115) and a gas diffusion layer (loaded with cathode catalyst) is employed to realize the gas-solid interface and promote cation transport (Note: hot pressing is required for sufficient contact).
• Electrode body 2 stands as the core component of the solid-state electrolyte cell, featuring a built-in 3mm thick square (4cm2) hollow layer. This layer can be filled with molded solid electrolyte, replacing the conventional liquid electrolyte, and enabling the direct preparation of liquid fuels. The solid electrolyte primarily consists of high-performance conductive polymers or
porous ceramics. The use of pure water aims to carry away the liquid product, facilitating a shift of the reaction equilibrium to the right and obtaining impurity-free product aqueous solutions. Substances generated in electrode body 1 and electrode body 3 pass through their respective membranes and react in intermediate electrode body 2, producing products carried away by flowing pure water.
Includes PEEK parts manufactured using Ensinger™ TECAPEEK (medical-grade)




Reviews
There are no reviews yet.